CBIS LATEST NEWS.
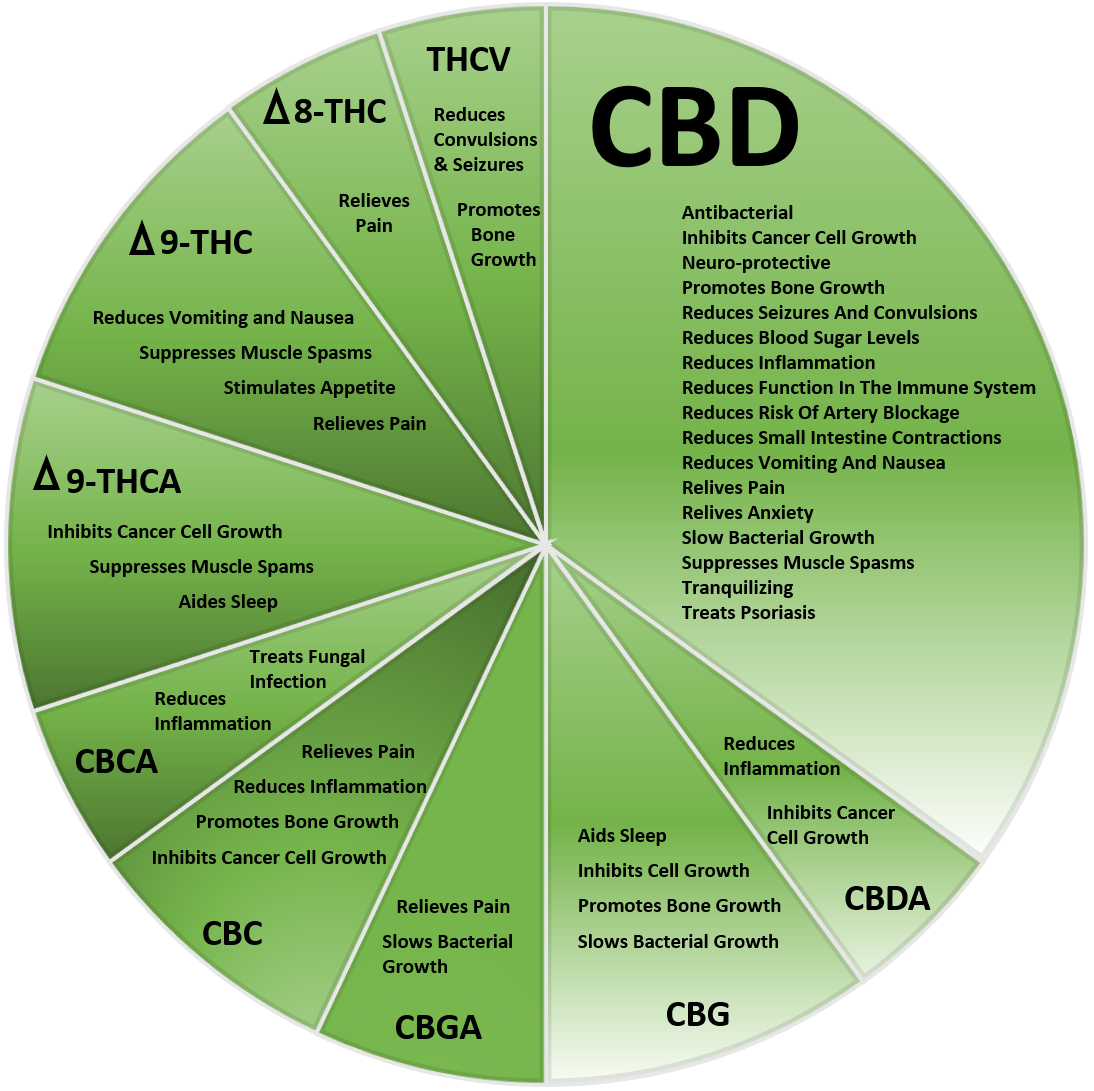
CRITICAL AILMENTS.
Cannabinoids act as bio-regulators and they have been recommended for an increasing variety of ailments. Scientific research has shown that CBD and THC found in cannabis may aid in treating a variety of medical conditions. Today, doctors prescribe medical cannabis as a treatment for issues such as chronic pain, eating and sleeping disorders linked to other medical treatments like chemotherapy.
Cannabis Science’s current laboratory research forms part of an aggressive drug development pipeline targeting critical ailments from laboratory research to scientific publication that Cannabis Science is developing with its research partners. This strongly demonstrates and effects the Company’s strong commitment to cutting-edge cannabinoid science in its pharmaceutical development programs. Cannabis Science has developed this pipeline to ensure that the Company’s research work reaches the scientific community and the patients in a timely fashion.
Cannabis Science’s funded research focuses on the clinical applications of cannabinoids in cancer treatment and pain management, but the company will expand their portfolio in the following significant directions:
- Investigate the effectiveness of cannabinoid treatment in an increasing array of cancers, especially difficult to treat cancers and those with high fatality rates,
- Develop novel targeted delivery methods in the use of cannabinoids, and
- With advances in bioinformatics, computer science, and pharmacology, Cannabis Science will explore the mechanisms of action of cannabinoids in the management of diseases
Cannabis Science’s priority has always been to identify unique approaches using cannabinoids in the treatment of a range of cancers and other critical ailments. There is a growing body of evidence that demonstrates the effectiveness of cannabinoids in the treatment of cancer. These pharmacologic compounds act on both CB-1 and CB-2 receptors and limit inflammation, curb cell proliferation, and affect cell survival. There have been a number of substantial reviews of the progress and promise of cannabinoids as anti-cancer drugs.
IRVINE, CA--(Marketwired - Jun 22, 2017) - Cannabis Science, Inc. (OTC PINK: CBIS), a U.S. company specializing in the development of cannabinoid-based medicines, is pleased to announce that its new product-development plans are well underway with the release of the first batch of the CBIS Transdermal Patch in response to an overwhelming response from hundreds chronic pain self-medicating patients worldwide with various chronic pain conditions including Neuropathy, Fibromyalgia, Diabetic Nerve Pain, Back Strains, and others.
"The CBIS medicated adhesive transdermal patch is placed on the skin to deliver a specific dose of cannabinoid medications into the bloodstream. Self-medicating patients have been reporting promoted healing effects to specific injured areas of the body experiencing chronic pain. An advantage of a transdermal drug delivery route over other types of medication delivery such as oral, topical, intravenous, intramuscular, etc. is that the patch can provide a controlled release of the medication into the patient, usually through a porous membrane covering a reservoir of medication or body heat that melts thin layers of high potency cannabinoid formulations embedded in the adhesive; the medication slowly enters the bloodstream at the chronic pain spot and then penetrates the central nervous system delivering the pain relief reported by self-medicating patients," said Dr. Allen Herman, Chief Medical Officer of Cannabis Science.
As well, CBIS continues to grow its distribution network in Los Angeles, California by adding Kush Factory as CBIS' newest Spotlight Dispensary for the month of July 2017.
Kush Factory is the latest dispensary to join the Cannabis Science new product distribution network and has received most of the recently released CBIS products, including the previously sold-out CBIS Metered Dose Inhalers (MDI) and the existing line of extracted tinctures, pills, sprays, balms, suppositories, and creams along with the upcoming release of more targeted controlled delivery modules.
As Spotlight Dispensary of the Month of July 2017, Kush Factory will be the first dispensary to carry the highly anticipated Chronic Pain CBIS Transdermal Patches.
The Kush Factory
612 N Vermont,
Los Angeles, California 90004
323-219-2827
This email address is being protected from spambots. You need JavaScript enabled to view it.
Please contact Kush Factory directly for ordering all CBIS products and delivery schedules.
The CBIS MDI's continue to be highly sought after and the company is working to increase production to meet strong demand from patients. The CBIS Unisex Suppository Regimen Package consists of rectal suppositories, and is the first of three regimen packages including a separate female and male package for self-medicating patients who prefer alternate delivery methods that will be released by CBIS.
About Cannabis Science, Inc.
Cannabis Science, Inc. takes advantage of its unique understanding of metabolic processes to provide novel treatment approaches to a number of illnesses for which current treatments and understanding remain unsatisfactory. Cannabinoids have an extensive history dating back thousands of years, and currently, there are a growing number of peer-reviewed scientific publications that document the underlying biochemical pathways that cannabinoids modulate. The Company works with leading experts in drug development, medicinal characterization, and clinical research to develop, produce, and commercialize novel therapeutic approaches for the treatment for illnesses caused by infections as well as for age-related illness. Our initial focus is on skin cancers, HIV/AIDS, and neurological conditions. The Company is proceeding with the research and development of its proprietary drugs as a part of this initial focus: CS-S/BCC-1, CS-TATI-1, and CS-NEURO-1, respectively.
Forward-Looking Statement
This Press Release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Act of 1934. A statement containing words such as "anticipate," "seek," intend," "believe," "estimate," "expect," "project," "plan," or similar phrases may be deemed "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Some or all of the events or results anticipated by these forward-looking statements may not occur. Factors that could cause or contribute to such differences include the future U.S. and global economies, the impact of competition, and the Company's reliance on existing regulations regarding the use and development of cannabis-based drugs. Cannabis Science, Inc., does not undertake any duty nor does it intend to update the results of these forward-looking statements. Safe Harbor Statement. The Private Securities Litigation Reform Act of 1995 provides a 'safe harbor' for forward looking statements. Certain of the statements contained herein, which are not historical facts are forward looking statements with respect to events, the occurrence of which involved risks and uncertainties. These forward-looking statements may be impacted, either positively or negatively, by various factors. Information concerning potential factors that could affect the company is detailed from time to time in the company's reports filed with the Securities and Exchange Commission.
CONTACT INFORMATION
-
Cannabis Science, Inc.
Dr. Allen Herman
Chief Medical Officer (CMO)
This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: 1-888-263-0832
Cannabis Science, Inc.
Jacques P. Walker
Member, International Government Affairs Board
This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: 1-888-263-0832
Cannabis Science, Inc.
Mr. Raymond C. Dabney
President & CEO, Co-Founder
This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: 1-888-263-0832
IRVINE, CA--(Marketwired - May 11, 2017) - Cannabis Science, Inc. (OTC PINK: CBIS), a U.S. company specializing in the development of cannabinoid-based medicines, announces the release of a limited batch of cannabinoid-based suppositories for self-medicating, male and female patients. The suppositories aid with symptoms related to cancer, anxiety, insomnia, and severe pain relief. This Unisex Suppository Regimen Package, consisting of rectal suppositories, is the first of three regimen packages that will be released by CBIS to address the tremendous demand for cannabinoid-based medicines for self-medicating patients who require alternative delivery methods.
Suppositories are 50% to 70% more efficient and produce a more predictable effect than smoked or ingested cannabis. The rectal endothelium provides greater bioavailability than transcutaneous and oral delivery of cannabinoids. Suppositories can also be more effective in treating pelvic and lower gastrointestinal conditions. Additionally, suppositories are effective in individuals with nausea and vomiting, those with difficulty in swallowing pills (the elderly), those with wasting conditions especially in cases where appetite is inhibited, and in individuals on chemotherapy who find it difficult to manage oral medications.
"This is another ground-breaking product release. We expect our suppository regimens to help address a significant demand for cannabinoid-based medicines targeting several patient-specific indications. Through this limited release of unisex regimens to self-medicating patients, we expect to track the use of these products through the dispensary, and observe the effectiveness of this particular formulation and delivery system. This process, as part of an overall observation and testing protocol, will enable CBIS to develop products that can help the largest number of patients possible," stated Dr. Allen Herman, CBIS' Chief Medical Officer.
To enroll in this observational study, please contact Moonlight Cannabis Dispensary, located at 5801 E. Beverly Boulevard, Suite 100, Los Angeles, CA 90022. The dispensary's telephone number is 323.834.9420, and the email address is This email address is being protected from spambots. You need JavaScript enabled to view it..
"The release of CBIS' Unisex Suppository Regimen is an important milestone as we build the company's portfolio of cannabinoid-based medicines. While this regimen is intended for men and women, we expect to release gender-specific suppositories and other products in the near future," stated Mr. Raymond C. Dabney, CBIS' President & CEO and Co-founder.
About Cannabis Science, Inc.
Cannabis Science, Inc. takes advantage of its unique understanding of metabolic processes to provide novel treatment approaches to a number of illnesses for which current treatments and understanding remain unsatisfactory. Cannabinoids have an extensive history dating back thousands of years, and currently, there are a growing number of peer-reviewed scientific publications that document the underlying biochemical pathways that cannabinoids modulate. The Company works with leading experts in drug development, medicinal characterization, and clinical research to develop, produce, and commercialize novel therapeutic approaches for the treatment for illnesses caused by infections as well as for age-related illness. Our initial focus is on skin cancers, HIV/AIDS, and neurological conditions. The Company is proceeding with the research and development of its proprietary drugs as a part of this initial focus: CS-S/BCC-1, CS-TATI-1, and CS-NEURO-1, respectively.
Forward-Looking Statements
This Press Release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Act of 1934. A statement containing words such as "anticipate," "seek," intend," "believe," "estimate," "expect," "project," "plan," or similar phrases may be deemed "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Some or all of the events or results anticipated by these forward-looking statements may not occur. Factors that could cause or contribute to such differences include the future U.S. and global economies, the impact of competition, and the Company's reliance on existing regulations regarding the use and development of cannabis-based drugs. Cannabis Science, Inc., does not undertake any duty nor does it intend to update the results of these forward-looking statements. Safe Harbor Statement. The Private Securities Litigation Reform Act of 1995 provides a 'safe harbor' for forward looking statements. Certain of the statements contained herein, which are not historical facts are forward looking statements with respect to events, the occurrence of which involved risks and uncertainties. These forward-looking statements may be impacted, either positively or negatively, by various factors. Information concerning potential factors that could affect the company is detailed from time to time in the company's reports filed with the Securities and Exchange Commission.
CONTACT INFORMATION
-
Cannabis Science, Inc.
Dr. Allen Herman
Chief Medical Officer (CMO)
This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: 1-888-263-0832
Cannabis Science, Inc.
Mr. Raymond C. Dabney
President & CEO, Co-Founder
This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: 1-888-263-0832
Cannabis Science, Inc.
Investor Relations
Teresa Misenheimer
This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: 1-888-263-0832
IRVINE, CA--(Marketwired - May 2, 2017) - Cannabis Science, Inc. (OTC PINK: CBIS), a U.S. company specializing in the development of cannabis-based medicines, is pleased to announce its President, CEO, and Co-Founder, Mr. Raymond C. Dabney, and his team have successfully participated in the Global Health Catalyst (GHC) Summit hosted by the Dana-Farber Cancer Institute/Harvard Cancer Center (DF/HCC). The DF/HCC is a collaborative organization that integrates five Boston-area academic medical institutions and two Harvard schools -- Beth Israel Deaconess Medical Center, Boston Children's Hospital, Brigham and Women's Hospital, Dana-Farber Cancer Institute, Massachusetts General Hospital, Harvard Medical School, and the Harvard T.H. Chan School of Public Health at Harvard Medical School. The summit is designed to catalyze high impact international collaborations in health care, education and research.
Mr. Dabney was the keynote speaker for the Cannabis Science versus Cancer and Other Malignancies Session, which was one of the highlights of the three-day Summit. CBIS' Chief Medical Officer, Dr. Allen Herman, Chaired the Cannabis Science Session, and also delivered a presentation on the epidemiology of cancer and the utilization of opioids in Africa and across the world during the Palliative Care and Mental Health Session. The Company is cleaning up the recorded video feed of the CBIS Session for enhanced clarity; distribution will be made available for viewing over the internet available shortly.
"We were pleased to share our vision with an impressive array of senior health care professionals from the USA and across the world including Africa and the broad African Diaspora. We were particularly pleased with our successful meetings with the Ministers of Health of Namibia, Rwanda, and Kisumu County in Kenya. These African health leaders will help CBIS to define our strategy on the African continent. We are launching an aggressive research program with Dana Farber, and we expect a number of major developments in 2017," said Dr. Herman.
Mr. Dabney stated, "Our participation in the GHC Summit and this speaking opportunity gave us an opportunity to discuss cutting-edge cannabinoid research with some of the greatest minds in modern medicine globally, as well as to network and explore potential partnerships." In addition, CBIS' participation in this Summit provided the company with an opportunity to update stakeholders regarding the progress of the implementation of CBIS' research agreement with Dana-Farber. "I believe the CBIS/Dana Farber relationship is off to a very good start, and I am excited about the potential of our collaboration. I am even more excited about the groundbreaking initiative with Dana-Farber that we plan to jointly announce in the next few days. This upcoming announcement will change the narrative of how we approach the research, development, and clinical trials of cannabinoid-based medicines globally."
About Cannabis Science, Inc.
Cannabis Science, Inc. takes advantage of its unique understanding of metabolic processes to provide novel treatment approaches to a number of illnesses for which current treatments and understanding remain unsatisfactory. Cannabinoids have an extensive history dating back thousands of years, and currently, there are a growing number of peer-reviewed scientific publications that document the underlying biochemical pathways that cannabinoids modulate. The Company works with leading experts in drug development, medicinal characterization, and clinical research to develop, produce, and commercialize novel therapeutic approaches for the treatment for illnesses caused by infections as well as for age-related illness. Our initial focus is on skin cancers, HIV/AIDS, and neurological conditions. The Company is proceeding with the research and development of its proprietary drugs as a part of this initial focus: CS-S/BCC-1, CS-TATI-1, and CS-NEURO-1, respectively.
Forward-Looking Statements
This Press Release includes forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Act of 1934. A statement containing words such as "anticipate," "seek," intend," "believe," "estimate," "expect," "project," "plan," or similar phrases may be deemed "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Some or all of the events or results anticipated by these forward-looking statements may not occur. Factors that could cause or contribute to such differences include the future U.S. and global economies, the impact of competition, and the Company's reliance on existing regulations regarding the use and development of cannabis-based drugs. Cannabis Science, Inc., does not undertake any duty nor does it intend to update the results of these forward-looking statements. Safe Harbor Statement. The Private Securities Litigation Reform Act of 1995 provides a 'safe harbor' for forward looking statements. Certain of the statements contained herein, which are not historical facts are forward looking statements with respect to events, the occurrence of which involved risks and uncertainties. These forward-looking statements may be impacted, either positively or negatively, by various factors. Information concerning potential factors that could affect the company is detailed from time to time in the company's reports filed with the Securities and Exchange Commission.
CONTACT INFORMATION
-
Cannabis Science, Inc.
Dr. Allen Herman
Chief Medical Officer (CMO)
This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: 1-888-263-0832
Cannabis Science, Inc.
Mr. Raymond C. Dabney
President & CEO, Co-Founder
This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: 1-888-263-0832
Cannabis Science, Inc.
Investor Relations
Teresa Misenheimer
This email address is being protected from spambots. You need JavaScript enabled to view it.
Tel: 1-888-263-0832

