Core Typography
Heading Text
h1. Heading Secondary text
h2. Heading Secondary text
h3. Heading Secondary text
h4. Heading Secondary text
h5. Heading Secondary text
h6. Heading Secondary text
TIPS: Create lighter, secondary text in any heading with a generic <small> tag or the .small class.
Example body text
This is a lead paragraph.
Make a paragraph stand out by adding .lead.
Maecenas sed diam eget risus varius blandit sit amet non magna. Donec id elit non mi porta gravida at eget metus.
Nullam quis risus eget urna mollis ornare vel eu leo. Cum sociis natoque penatibus et magnis dis parturient montes, nascetur ridiculus mus. Nullam id dolor id nibh ultricies vehicula.
The following snippet of text is rendered as bold text.
The following snippet of text is rendered as italicized text.
An abbreviation of the word attribute is attr.
Address
Company Name795 Folsom Ave, Suite 600
San Francisco, CA 94107
P: (123) 456-7890Full Name
P: (123) 456-7890
first.last@example.com
Emphasis classes
This is a .mute paragraph.
This is a .text-primary paragraph.
This is a .text-warning paragraph.
This is a .text-danger paragraph.
This is a .text-success paragraph.
This is a .text-info paragraph.
Alignment classes
This is a left aligned text .text-left
This is a center aligned text .text-center
This is a right aligned text .text-right
This is a justify aligned text which is often used in Book Design, Magazine or special Typo Pages. Create a justify aligned text with .text-justify class.
Blockquotes
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer posuere erat a ante.
Quote's author in Source Title
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Integer posuere erat a ante.
Quote's author in Source Title
Tags
Color Swatches
Brand Colors
- #00bfe6
- Brand Primary
- #428bca
- Brand Secondary
- #f0ad4e
- Brand Warning
- #fbe57c
- Brand Danger
- #5bc0de
- Brand Info
- #4db18d
- Brand Success
Grayscale Levels
- #262626
- Gray Darker
- #3c3c3c
- Gray Dark
- #555555
- Gray
- #999999
- Gray Light
- #eaeaea
- Gray Lighter
- #f7f7f7
- Gray Lightest
Navigations
Inputs
Indicators
Alerts and Notifications
error message title
Donec eget purus nec tellus tincidunt lacinia et sed lorem. Cras ac dignissim mauris. Duis odio diam, rutrum ut est at, scelerisque malesuada risus.
sussess message title
Donec eget purus nec tellus tincidunt lacinia et sed lorem. Cras ac dignissim mauris. Duis odio diam, rutrum ut est at, scelerisque malesuada risus.
info message title
Donec eget purus nec tellus tincidunt lacinia et sed lorem. Cras ac dignissim mauris. Duis odio diam, rutrum ut est at, scelerisque malesuada risus.
warning message title
Donec eget purus nec tellus tincidunt lacinia et sed lorem. Cras ac dignissim mauris. Duis odio diam, rutrum ut est at, scelerisque malesuada risus.
Badges
Labels
Misc
List groups
- 14 Cras justo odio
- 2 Dapibus ac facilisis in
- Morbi leo risus
- 1 Morbi leo risus
- 2 Dapibus ac facilisis in
Panels
Panel primary
Panel success
Panel warning
Panel danger
Panel info
Wells
INVESTOR INFORMATION.
Cannabis Science alleviates suffering and promotes health through cannabinoid science. A pioneering biotech company, Cannabis Science creates cannabinoid-based extract formulations for various medical conditions, including more than 8 different types of Cancer.
Each individual one of these formulations has revenue and earnings potential in the hundreds of millions of dollars based on the current global pharmaceutical industry. The potential revenue and earnings are combined with additional profit centers including an educational model, nutraceutical products, and a strategic partnership with MedBox® Solutions. These additional profit centers bring a higher level of confidence to realizing our overall financial strategy and plan to bring Cannabis Science into positive earnings per share by 2015.
The cannabinoid market and industry is best explained using the alcohol prohibition black market model in the 1920’s, and then as a pharmaceutical retail market in the 1930’s.
The cannabinoid market is estimated at $30-$50 billion. Internal estimates raise that number to $74 billion upon speculation of the Federal Medical Cannabis Program in 2017.
Cannabis Science's President Emeritus, Dr. Robert Melamede, Ph.D., is focused on the quantum physics relationship with biology, and the role the cannabinoid strain plays in the string of life. His research and science of the cannabinoid system along with the relationship between the endocannabinoid system and phytocannabinoids is pioneering. The Cannabis Science Scientific Advisory Board is comprised of several doctors who embrace and support Dr. Melamede’s vision. Board members are all considered global leaders.
Cannabis Science's executive management team is built through a partnership of independent consultants whose interests are aligned with the success of shareholders and Cannabis Science. This model and their executive management plan have allowed Cannabis Science to attract strategic partnerships that generate revenues, as with Vincent Mehdizadeh, from MedBox. It also has allowed Cannabis Science to strengthen the services of the company both with investors through Robert Kane, Investor Relations Manager, and with patients.
Cannabis Science is the first publicly traded cannabinoid company on the market, whose symbol is CBIS, and is the only publicly traded company in existence that is involved in cannabinoid-extract based formulations. This model and leadership position is of great value as the company matures over the next twenty years and expands globally into a potential billion dollar biotech global leader.
In conclusion, Cannabis Science is a company doing the right things, for the right reasons, with the right people, a company started by someone who was ill and found a way to heal themselves. That person shared their solution with others and they have shared with hundreds of millions of patients who are suffering; chronic pain patients, cancer patients, MS patients, soldiers and veterans with PTSD, those who are terminally ill, and patients with dozens of other conditions are now all aware of the medicinal value of cannabinoid. Now is simply the best opportunity for Cannabis Science to help and heal those who are suffering through their formulations and services.
{rsform 1}
]]>Cannabis Science’ proprietary, short-time vacuum distillation process determined the ideal pressure, temperature, and time to avoid the loss of CBDA and all of the important acids of cannabinoids. As part of its testing process, the Company utilized CBD extract with 7.3% CBD and less than 0.2% THC levels. Through the first step of CBIS’ purification process, the Company produced a purified CBD extract with 26% CBD and over 0.6% THC levels. Based on this cleaned extract Cannabis Science EU GmbH produced a purified water-soluble CBD powder with 3.5% CBDA, 3.5% CBD, and less than 0.02% THC levels. Generally, cannabinoids become unstable when mixed with water due to the oxidation process. Through the Company’s vacuum distillation process and emulsification solution, CBIS can provide its customers with the highest CBDA extracts in powder form, thereby bypassing this oxidation and providing a potent product that maintains full nutritional CBDA and CBD levels with a much longer consumption expiry date than in water-diluted common CBD products, commonly poor in CBDA.
{rsform 6}
]]>Welcome to your “Personal Loyalty Gift” from Mr. Raymond. C. Dabney This is a very special time and I want to extend my personal “Thanks” to everyone who has supported me throughout the years. I have been blessed with “Very Loyal People” who have supported me for years on end. I will recognize your names, and I look forward to reconnecting with many of you via phone. I have lost contact with some of you and you know who you are, I will be reaching out to you as well. Each of you are included in this “Personal Thank You Gift”, whether you own CBIS Shares or not. Again Thank YOU All, for your confidence, your support, and your Loyalty throughout the years. For this Loyalty I will be “Giving you some of my personal shares” of Cannabis Science Inc. This is my personal confirmation of my Appreciation of you and your Loyalty. To the CBIS shareholders that I will “Gift some of my Shares”, I want and expect nothing in return, as a matter of fact, in the future everyone can look forward to “more Loyalty Gifts” as we progress forward with our High-Growth Business Models in our group of Companies.
Loyalty is a SPECIAL word, and I value it as such. Finding Loyal people for “your/any team” is a very difficult task and I value Greatly the ones I have. Unfortunately, sometimes people are not loyal and have their own deceitful strategies to unhinge you from your path of success. It is ironic how things do work out, when faced with such adversaries. Our Loyal people in our lives seem to rise up and shine in these moments “to fight the deceptions” they seem to fight with the Wrath of God to protect our Companies and all the Loyal individuals in our lives.
So with this in mind, I definitely want to be able to reward the “Loyal people in our Lives” the ones who stand beside you, who support you, protect you, and lift you up by any means they can, in anyway they can. As I am sure you can imagine, I am just as excited as you are to process these “Gifts” and give them to you as quickly as possible. We appreciate your patience as we process your “Loyalty Gift” and my legal team assesses the simplest mechanism we can use to accomplish our goal.
To initiate your “Loyalty Gift” please fill out the required (*) fields below:
Thanks Again for your Loyalty,
Talk soon.
Mr. Raymond C. Dabney
Director, President & CEO, Co-Founder
Cannabis Science, Inc.
raymond.dabney@cannabisscience.com
Tel:1-888-263-0832
{rsform 5}
]]>
Cannabis Science, Inc. takes advantage of its unique understanding of metabolic processes to provide innovative treatment options for unmet medical needs.
Cannabis use has an extensive history dating back thousands of years, and currently there are thousands of peer-reviewed scientific publications that document the underlying biochemical pathways that cannabinoids modulate.
At Cannabis Science, we use an inquiring approach to discover and develop novel cannabinoid-based therapies to improve patients’ lives. From the initiation, our founders have been committed to fostering and maintaining a bold, pioneering spirit fostering the true nature of innovation from which cutting edge ideas flourish and translate into evidence-based solutions.
We are dedicated to working closely with local, national and international regulatory agencies to provide access to high quality, first class cannabinoid pharmaceuticals to those critically in need of new treatments for life threatening and debilitating conditions. Cannabis Science’s clinical trial material comes from the cultivation and production facilities that are cGMP compliant, surpassing high quality standard industrial and food processing requirements.
The Company works with leading experts in drug development, medicinal characterization, and clinical research to develop, produce, and commercialize novel therapeutic approaches for the treatment of multiple critical ailments from cancer and infections to age-related illnesses and neurobehavioral disorders.
Our products, broadly described, are medical cannabinoid formulations developed from one or more of the cannabinoid compounds found in the cannabis plant. Our immediate focus is to treat one of the most important diseases in the world, cancer.
In 2013, Cannabis Science submitted patent application N2010968 in Europe entitled "Composition for the Treatment of Neurobehavioral Disorders" (CS-NEURO-1). The subject of the patent is development of cannabinoid-based formulations to treat a variety of neurobehavioral disorders.
BASIS & PHILOSOPHY
Cannabis plants have extensive history of medical and agricultural use dating back thousands of years.
To date hundreds of natural constituents covering several chemical classes have been isolated and identified from the Cannabis plant.
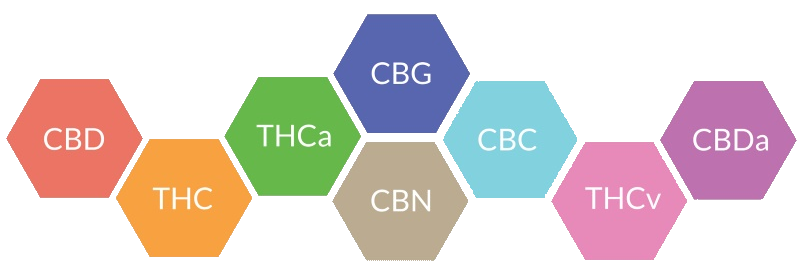
SOME KEY PHYTOCANNABINOIDS ARE:
-
tetrahydrocannabinol (THC)
-
cannabidiol (CBD)
-
cannabigerol (CBG)
-
cannabichromene (CBC)
-
cannabinol (CBN)
These cannabinoids belong to the chemical class of terpenophenolics, of which 85 have been uniquely identified in cannabis, including the most psychoactive cannabinoid, THC. Some applications of cannabinoids have been well established in peer-reviewed literature such as for alleviating nausea and stimulating the appetite for people with AIDS and cancer. Other well-known uses include easing chronic pain and reducing muscle spasms associated with multiple sclerosis and spinal cord injuries.
The pharmacology of THC has been widely studied, while many other identified cannabinoids are still poorly characterized pharmacologically and biologically, with new activities for cannabinoids consistently being discovered.
Cannabis Science is developing novel cannabis based approaches to treat the world’s most deadly illnesses. We learn from patients about the healing properties of cannabis medicines. Our immediate focus is the development of cutting edge cancer treatments.
The Company’s future endeavors include infectious illnesses, neurobehavioral disorders including attention deficit disorder, post-traumatic stress disorder; and an application of the anti-inflammatory activities of cannabis in the management of age-related illnesses.
The endocannabinoid system possessed by all vertebrates regulates all body systems and maintains homeostasis. As such, the mechanisms of phytocannabinoids’ biological impact are multidimensional.
While concentrating on our core activities of discovering and developing treatments that will make a meaningful difference in patients’ lives, we remain mindful that we have other responsibilities to the clinicians who utilize our drugs, health authorities around the world, our shareholders, our employees, and the communities in which we live and work. We continually strive to improve our corporate responsibility standards and activities, implementing comprehensive ethical standards and undertaking patient and community progressive initiatives.
These principles reflect the mission of Cannabis Science to provide innovative therapeutics for unmet medical needs.
As the industry leader, Cannabis Science consults and leads other emerging businesses that Cannabis Science believes has a preferred business model, one which will mature into a key business model in the future. Our consulting is on the entire "seed to sale" process with a focus on bio-pharmaceutical development.
Cannabis Science is one of the longest standing companies in the cannabis business.
We feel that the correct way is to look at the industry from a bio-pharmaceutical standpoint, in a manner that allows cannabinoid-based products to modulate the endocannabinoid system to treat multiple conditions. Scientifically, we know this is the beginning of one of the greatest expansions of medicine and industry we will ever experience in our lifetime.
]]>
THE SCIENCE OF CANNABINOIDS.
Cannabinoids have an extensive history dating back thousands of years, and currently, there are thousands of peer-reviewed scientific publications that document the underlying biochemical pathways that cannabinoids modulate. The endocannabinoid system possessed by all vertebrates regulates all body systems and maintains homeostasis. As such, the mechanisms of phytocannabinoids’ biological impact are multidimensional.
Some applications of cannabinoids have been well established in peer-reviewed literature such as alleviating nausea and stimulating the appetite for people with AIDS and Cancer. Other well-known uses include easing chronic pain and reducing muscle spasms associated with neuromuscular disorders like MS and spinal cord injuries. Some current uses are poorly understood, such as its perceived effectiveness in alleviating certain autoimmune disorders, such as Crohn’s Disease.
Our team of scientists will produce and commercialize products derived from cannabis-based botanical extracts. Even today, new uses for cannabinoids are consistently discovered. For example, scientists recently found that topical cannabinoid-based preparations can be effective against MRSI, the deadly antibiotic-resistant flesh-eating disease. Other topical applications, which are largely non-psychoactive, would target localized pain, such as arthritis and burns, as well as neuropathic pain, for which there are few effective treatments.
In 2001, an article published in the Canadian Medical Association Journal revealed that, based on self-identified needs, it is estimated that 2% (or 400,000) of Canadian adults were already utilizing cannabinoids for medical purposes. With ever increasing use and scientific support for cannabinoid medicines occurring in the US and around the world, we are looking at a new multi-billion dollar industry that will play a dramatic role that will impact human health on a global scale.

CANNABINOIDS OVERVIEW
Cannabis sativa is one of the most widely used plants for both recreational and medicinal purposes. To date a total of 525 natural constituents covering several chemical classes have been isolated and identified from Cannabis sativa. The cannabinoids belong to the chemical class of terpenophenolics, of which 85 have been uniquely identified in cannabis, including the most psychoactive cannabinoid, tetrahydrocannabinol commonly referred to as THC.
The most common natural plant cannabinoids (phytocannabinoids) are: THC, cannabidiol (CBD), cannabigerol (CBG), cannabichromene (CBC), and cannabinol (CBN). Several of the identified cannabinoids are both chemically and pharmacologically poorly characterized; however, the pharmacology of THC has been widely studied, and it is regarded as the main psychoactive constituent of cannabis.
Cannabinoids were first discovered in the 1940s, when CBD and CBN were identified. THC was not identified until 1964, but by that time cannabinoids had been removed from the pharmacopeiae of most countries, making further research on the plant difficult.
There are three general types of cannabinoids:
- Phytocannabinoids, that occur uniquely in the cannabis plant.
- Endogenous cannabinoids produced naturally in many animal species including humans.
- Synthetic cannabinoids compounds produced in a laboratory. Forms of synthetic THC are available by prescription in a number of countries, including the US. In the US, marketed as Marinol®.
Phytocannabinoids, also called natural cannabinoids, herbal cannabinoids, and classical cannabinoids, are only known to occur naturally in significant quantity in the cannabis plant. They are concentrated in a viscous resin that is produced in glandular structures known as trichomes, and are most prevalent in the flowers of the female plants.
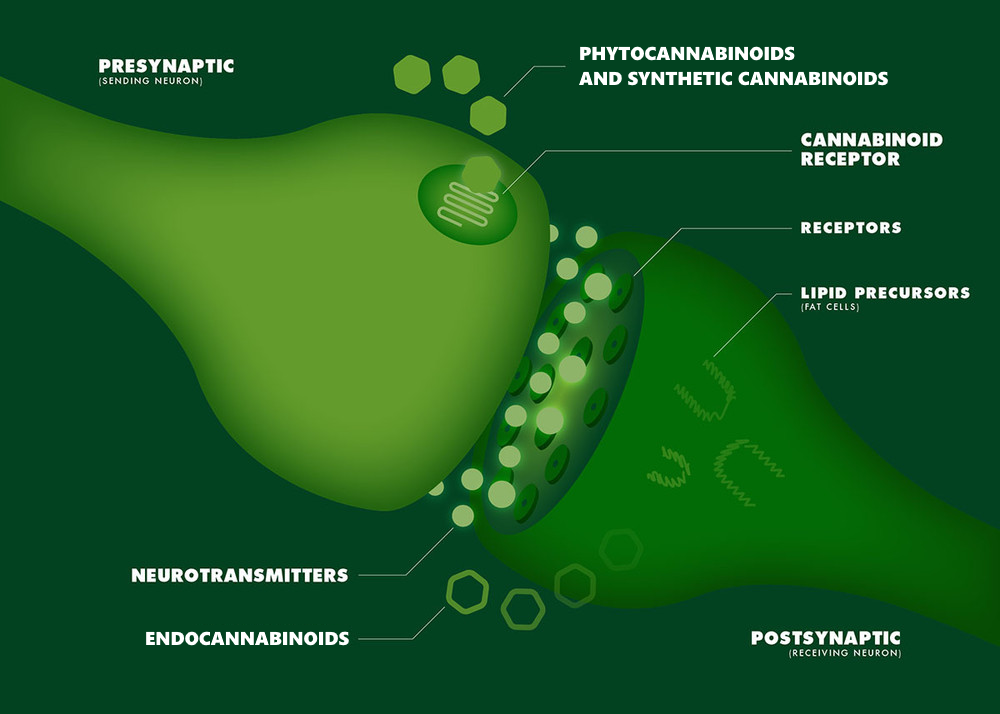
ENDOCANNABINOID SYSTEM
The endocannabinoid system refers to a group of neuromodulators and receptors involved in a variety of physiological processes including appetite, pain sensation, mood, and memory. The system is named for endocannabinoids, the endogenous lipids that bind cannabinoid receptors.
CB1 receptors are found throughout the body and are the most prevalent neurotransmitter system in the brain, specifically in the basal ganglia and in the limbic system, including the hippocampus. They are also found in the cerebellum and in both male and female reproductive systems.
CB2 receptors are found most exclusively in the immune system, with the greatest density in the spleen. CB2 receptors appear to be responsible for the anti-inflammatory and possibly other therapeutic effects of cannabis. CB2 receptors appear in any tissue when there is pathology.
Science increasingly recognizes the role that endo-cannabinoids play in major life functions in the human body. Cannabinoids act as a bio regulatory mechanism, which explains why they have been recommended as a treatment for many diseases and ailments in anecdotal reports and scientific literature. Common prescribed ailments include: Pain, arthritic conditions, migraine headaches, anxiety, epileptic seizures, insomnia, loss of appetite, GERD (chronic heartburn), nausea, glaucoma, AIDS wasting syndrome, depression, bipolar disorder (particularly depression-manic-normal), multiple sclerosis, menstrual cramps, Parkinson's, trigeminal neuralgia (tic douloureux), high blood pressure, irritable bowel syndrome, and bladder incontinence.
Cannabinoids have been used medicinally for thousands of years in China, India, and the Middle East. Of course, most traditional medicines were plant-based, but until recently no one knew how or why they worked. Though cannabinoids are a very old form of medication, we are inspired by this "folk medicine," whose efficacy modern science now routinely verifies, and we are capable of creating systematic and effective use of cannabinoid therapies.
While cannabinoids were widely used in the West during the 19th century, the technology was lacking to detect and understand its active ingredients, to standardize dosages of those ingredients, and to effectively deliver those ingredients, owing largely to cannabinoids' non-water-solubility. Consequently, it became less widely used in the early 20th century and was eventually renamed "marijuana". It was gradually removed from the pharmacopeiae of most advanced countries; and research was discouraged.
]]>

Raymond C. Dabney
- President & CEO, Co-Founder and Director
Mr. Raymond C. Dabney, President & CEO, brings 25 years of experience in corporate finance, corporate structure, corporate communications, sales and marketing for public companies. Mr. Dabney has significant experience in public and private venture capital, early stage equity/debt financings and regulatory compliance.
Mr. Dabney has co-founded several public and private companies in many diverse industries such as drug development, entertainment, media, advertising, mining, automobile, high-tech, manufacturing, real estate and communications, many of which he launched from day one. Cannabis Science is a prime example of the type of organizations that Mr. Dabney has built from inception. Mr. Dabney has extensive international relationships that he has utilized to grow each Company, from the US, Canada, several European centers, such as Paris, London, Amsterdam and eastern Spain, as well as countries in the West Indies, South America, and Asia. Mr. Dabney’s niche market knowledge, team leadership, and management style facilitate the continued growth and forward thinking that successfully bring his projects from concept to completion.
 Allen A. Herman, M.B., Ch.B., Ph.D.
Allen A. Herman, M.B., Ch.B., Ph.D.
- Chief Medical Officer
Dr. Allen Herman is an epidemiologist and public health physician. He was the founding Dean of the National School of Public Health, Medical University of Southern Africa of the Republic of South Africa. He graduated in Medicine from the University of Natal in 1977 and completed his doctoral work in Epidemiology at the University of the Witwatersrand in 1989. He was a postdoctoral fellow in Epidemiology at Columbia University in New York in 1986 and a member of the faculty from 1987 to 1988. From 1989 to 1997 he was a Visiting Scientist at the National Institutes of Health. He was an Adjunct Professor at the George Washington University School of Public Health and Health Services from 1997 to 2004. Dr. Herman has extensive experience in developing and managing epidemiologic and public health research projects, and he is adept at secondary data analysis. In 1990 he helped develop the Baltimore Project, an Infant Mortality Reduction Initiative in Baltimore, Maryland. This community-based, model enriched prenatal care demonstration project for East Baltimore formed part of the basis for a $160 million dollar federally funded national program to reduce infant mortality. In 1992 he developed the scientific basis of the National Institutes of Health - District of Columbia Infant Mortality Reduction Initiative. He was the scientific director of this $25 million community-based U.S. federal research project that was designed to identify the critical factors that contribute to a high infant mortality among poor inner city African Americans and to develop interventions to reduce infant mortality rates.
In 1990 he was a member of the steering committee of a consortium of research programs for maternally-linked data from: the National Institutes of Health, the Centers for Disease Control and Prevention, the University of Oxford, the Australian Institute of Child Health, the Israeli Central Bureau of Statistics, and the University of Bergen, Norway. Maternally-linked data were created for several million births by linking birth data of siblings from the same mother into sibships. In the absence of national identification numbers that would allow deterministic linkage of birth records, Dr. Herman and his colleagues developed probabilistic linkage algorithms to create sibships for the states of Georgia, Washington, Missouri, and Utah. The work of the consortium culminated in an International Symposium on Maternally Linked Pregnancy Outcomes held in September 1995 in Atlanta, GA and a special issue of the journal Paediatric and Perinatal Epidemiology edited by Melissa Adams of the CDC, Allen Herman, and Francis Notzen of the National Center for Health Statistics.
From 2008 to 2013 Dr. Herman worked in Tennessee and Mississippi developing and implementing community oriented primary care based diabetes self management education (DSME) programs. He worked with federally qualified health centers (FQHCs) and private sector primary care providers to DSME to underserved communities in western Tennessee and Mississippi. These were among the initial Every One with Diabetes Counts programs of the Centers for Medicare and Medicaid Services and established the effectiveness of group based DSME interventions for Medicare beneficiaries.
Click here to read Dr. Herman's Curriculum Vitae
 Benjamin C.K. Tam
Benjamin C.K. Tam
- CFO and Director
Benjamin C.K. Tam was a contract controller for a chain of grocery stores since May 2013. Previously he served as chief financial officer of Ever88 Entertainment N.V. from 2010 to 2013, chief financial officer of CGTV Games Limited from 2006 to 2010, chief financial officer of Digifonica Canada Limited and Molaris Corp. from 2005 to 2006, public accountant and consultant from 1996 to 2005, chief financial officer/chief executive officer and director of Darius Technology Ltd from 1987 to 1996, and vice-president of finance and director of Bulldog Bag Ltd from 1978 to 1987.
Benjamin has been assisting the filing of financial reports of Cannabis Science since December 2015. He is a member of Chartered Professional Accountants (CPA) / Certified General Accountant (CGA) and obtained his CGA through University of British Columbia. He also holds a diploma of Business Administration from Lethbridge Community College, Alberta.
 Mario Lap
Mario Lap
- President of European Operations and Director
Mr. Lap brings to the Company a unique combination of expertise in Dutch and greater European medical cannabis law and policy as well as in the areas of health care, IT security, law enforcement, and government relations. Mr. Lap has written numerous international papers and legislative initiatives regarding medical and non-medicinal cannabis laws and access throughout Europe. Mr. Lap has made several television and radio appearances on CNN, BBC, France 2, RTL, and various Dutch public and commercial channels to give public education and information on policy, laws, and current medical breakthroughs.
Mr. Lap, a Dutch health and IT regulatory lawyer in Amsterdam, has extensive experience in Europe with health care and drug policy, medical cannabis regulation, substance use policy, IT project management, digital security policy, food and medicine regulation, and other governmental and private sector consulting. Mr. Lap currently serves as Director, Foundation on Drug Policy and Human Rights, and Director, Drugtext Foundation, and as a Director of various private ventures. Mr. Lap also served as Director of Sales of Apcare B.V., Director of Yalado International B.V., Founder and Director, Calyx Internet B.V. and Calyx Internet Corporation, Professor at the Rechercheschool (Dutch National Police Academy), Head of the Legal and Policy Department of the Netherlands Institute on Alcohol and Other Drugs, and Head of Legal and Policy Department, and Pilot Project of European Monitoring Center on Drugs and Drug Addiction.
Mr. Lap, has an extensive educational background through studies at the Het Amsterdams Lyceum, Colloqium Doctum University of Amsterdam, University of Amsterdam Law School, Open University and University of Liverpool, and speaks fluent Dutch, English, French, and German.
 Robert Kane
Robert Kane
- Chief Operation Officer and Director
Mr. Kane wrote his first business plan in 1996. In 1998, he was hired at Ryan, Beck, and Company, which was bought by the 120-year-old, top ten, financial firm Stifel Nicolaus (NYSE: SF) as a registered representative. On December 31, 2009, Mr. Kane walked away from the financial markets and started his own firm, Robert Kane Partners, which specializes in business and management planning, investor presentations, and investor relations. Mr. Kane held the position of Director of Investor Relations for Medical Marijuana, Inc., the first ever publicly traded medical marijuana company. He also held the position of Chief Financial Officer for “Cannabiz Business University.” Robert Kane's mission is transforming and leading the Medical Cannabis Industry into a viable investment alternative for all types of investors.
 Greta Gaines
Greta Gaines
- Product & Marketing Specialist
Greta Gaines is a leading advocate on the reform of cannabinoid law, spending years becoming an authority while serving on the Board of Directors of the National Organization to Reform Marijuana Law (NORML); the Board of the NORML Women's Alliance Foundation (as a Founding Member); and the Advisory Board of Patients Out of Time. Ms. Gaines is well known as an innovator in the use of hemp and developer of cannabinoid-based solutions as a valuable and healing commodity.
]]>
 Roscoe M. Moore, Jr., D.V.M., Ph.D., D.Sc.
Roscoe M. Moore, Jr., D.V.M., Ph.D., D.Sc.
- Chair of Scientific Advisory Board
Retired United States Assistant Surgeon General (Rear Admiral, USPHS) within the Immediate Office of the Secretary. Until his retirement, Dr. Roscoe M. Moore, Jr. served with the United States Department of Health and Human Services (HHS) and was for the last twelve years of his career the principal person responsible for global development support within the Office of the Secretary, HHS, with primary emphasis on Continental Africa and other less developed countries of the world. He was the principal liaison person between the HHS and Ministries of Health in Africa with regard to the development of infrastructure and technical support for the delivery of preventive and curative health needs for the continent.
Dr. Moore received his Bachelor of Science and Doctor of Veterinary Medicine degrees from Tuskegee Institute; his Master of Public Health degree in Epidemiology from the University of Michigan; and his Doctor of Philosophy degree in Epidemiology from the Johns Hopkins University. He was awarded the Doctor of Science degree (Honoris Causa) in recognition of his distinguished public health career by Tuskegee University.
Dr. Moore was a career officer within the Commissioned Corps of the United States Public Health Service (USPHS) entering with the U.S. National Institutes of Health (NIH) and rising to the rank of Assistant United States Surgeon General (Rear Admiral, USPHS) within the Immediate Office of the Secretary, HHS. He was selected as Chief Veterinary Medical Officer, USPHS, by Surgeon General C. Everett Koop.
Dr. Moore served as an Epidemic Intelligence Service Officer with the U.S. Centers for Disease Control and Prevention (CDC). He was with the Center for Veterinary Medicine, U.S. Food and Drug Administration (FDA), before becoming Senior Epidemiologist within the National Institute for Occupational Safety and Health, CDC. He served as the Chief Epidemiologist with the Center for Devices and Radiological Health, FDA. He directed the Epidemiology and Biostatistics Program and was an Assistant Professor of Oncology within the Howard University College of Medicine Cancer Center.
 Allen A. Herman, M.B., Ch.B., Ph.D.
Allen A. Herman, M.B., Ch.B., Ph.D.
Dr. Allen Herman is an epidemiologist and public health physician. He was the founding Dean of the National School of Public Health, Medical University of Southern Africa of the Republic of South Africa. He graduated in Medicine from the University of Natal in 1977 and completed his doctoral work in Epidemiology at the University of the Witwatersrand in 1989. He was a postdoctoral fellow in Epidemiology at Columbia University in New York in 1986 and a member of the faculty from 1987 to 1988. From 1989 to 1997 he was a Visiting Scientist at the National Institutes of Health. He was an Adjunct Professor at the George Washington University School of Public Health and Health Services from 1997 to 2004. Dr. Herman has extensive experience in developing and managing epidemiologic and public health research projects, and he is adept at secondary data analysis. In 1990 he helped develop the Baltimore Project, an Infant Mortality Reduction Initiative in Baltimore, Maryland. This community-based, model enriched prenatal care demonstration project for East Baltimore formed part of the basis for a $160 million dollar federally funded national program to reduce infant mortality. In 1992 he developed the scientific basis of the National Institutes of Health - District of Columbia Infant Mortality Reduction Initiative. He was the scientific director of this $25 million community-based U.S. federal research project that was designed to identify the critical factors that contribute to a high infant mortality among poor inner city African Americans and to develop interventions to reduce infant mortality rates.
Dr. Herman was effective in harnessing the energies of South Africans and Americans to fight HIV/AIDS in Africa. He led the development of the Bristol-Myers Squibb Company Secure the Future Project, a $100 million program that focused on the health of women and children infected and affected by HIV. He was the Advisor to then Chairman Ronald V. Dellums, who served President Bill Clinton (during his second term) as the Chairman of the U.S. Presidential Committee on HIV/AIDS on HIV/AIDS in Africa. Dr. Herman, Mr. Dellums and Congresswoman Barbara Lee (the founding cochair of the Congressional HIV/AIDS Caucus) developed the idea of an “AIDS Marshall Plan for Africa” which was transformed into the Global Fund to fight AIDS, Tuberculosis and Malaria, which led in 2003 to the President’s Emergency Plan for AIDS Relief (PEPFAR) and its reauthorization in 2008. Dr. Herman was the director of the Secure the Future Public Health Fellowship Program that trained more than 150 fellows in dealing with AIDS. This is the largest such training program in Africa, and his students included the Minister of Health of Swaziland, the Director of the AIDS Program of Lesotho, and the Directors of the AIDS Programs from a number of South African provinces. He also worked as the advisor to the South African National Defense Force and helped bring a substantial antiretroviral treatment program to the South African military. This program was designed to bring treatment through the use of clinical trials to a large population of military families and was designed to enable the South African government to objectively create programs of treatment for the large number of infected South Africans. For his work in HIV/AIDS, Dr. Herman was awarded the Heroes in Medicine award by the International Association of Physicians in AIDS Care.
Click here to read Dr. Herman's Curriculum Vitae
 Julius Garvey, M.D., F.A.C.S, F.R.C.S.(C), F.I.C.S., F.A.C.Ph., F.A.C.C.W.S.
Julius Garvey, M.D., F.A.C.S, F.R.C.S.(C), F.I.C.S., F.A.C.Ph., F.A.C.C.W.S.
Julius Garvey, M.D., F.A.C.S, F.R.C.S.(C), F.I.C.S., F.A.C.Ph., F.A.C.C.W.S. is a Board-Certified surgeon specializing in the diagnosis and treatment of vascular diseases. As the founder and Medical Director of Garvey Vascular Specialists, Dr. Garvey brings a wealth of experience and knowledge into our practice. His skill and proficiency provide our patients with an extensive range of expertise in the areas of arterial, venous diseases.
Dr. Garvey, a highly trained and credentialed surgeon, has held extensive academic appointments. He has worked as an Associate and Assistant Professor of Surgery at Albert Einstein College of Medicine in Bronx NY, Assistant Professor of Surgery, State University of NY at Stony Brook, NY, as well as Instructor in Surgery at both Albert Einstein College of Medicine in Bronx, NY and Columbia University College of Physicians & Surgeons, NY. Additionally, his hospital appointments are expansive. He has been an Attending Cardiothoracic Surgeon at Harlem Hospital Center in NY, NY and Montefiore Hospital in Bronx, NY, Associate Attending Head of Thoracic Surgery at the Montefiore Morrisania Affiliate in Bronx, NY, and Acting Program Director in the Division of Cardiothoracic Surgery at Long Island Jewish Medical Center in New Hyde Park, NY. He was also Chief of Vascular & Thoracic Surgery at Queens Hospital Center and is presently an attending surgeon on staff of the Long Island Jewish Medical Center.
Dr. Garvey is both nationally and internationally recognized for his contributions to medicine and speaks, teaches and presents clinical research at conferences across the country. He is a Member of the International College of Angiology, the Phlebology Society of America, the NY Academy of Sciences, the American Heart Association, the Society of Thoracic Surgeons, the Association for Academic Surgery, the Association for the Advancement of Wound Care and the NY Society of Thoracic Surgery.
Dr. Garvey is a Fellow of the Royal College of Surgeons (Canada), the American College of Surgeons, The American College of Phlebology, the International College of Surgeons, and the American College of Chest Physicians. In addition, he is a Diplomate of the Board of Cardiothoracic & Vascular Surgery, the American Board of Surgery, and the American Academy of Wound Management. Dr. Garvey’s exposure and expertise in vascular surgery, cardiovascular surgery, Phlebology, and wound care leads his determination to comprehensively address the concerns of all patients seen at Garvey Vascular Specialists afflicted with vascular disorders.
Click here to read Dr. Garvey's Curriculum
 Julia Royall
Julia Royall
Ms. Royall is a Member of the Scientific Advisory Board. As a specialized consultant, she will provide advice and expertise on health information databases, management systems, and Internet technology. Julia Royall is a leader in health information and has been working in international health in Africa since 1990, with more than 40 years of professional experience in the communications field. Julia’s commitment is to bring together information and technology with partners, projects and funding, using a variety of media.
Ms. Royall was recruited to the National Library of Medicine (NLM) at the National Institutes of Health (NIH) in 1997 to create a malaria research communications network (MIMCom) to support scientists in Africa as part of the Multilateral Initiative on Malaria. The first network of its kind, MIMCom comprised 27 research sites in 14 African countries and engaged over 30 partner organizations and institutions in the US, UK, Europe, and Africa.
As Chief of NLM’s Office of International Programs, she created innovative programs which focused on Africa and comprised outreach to medical librarians, medical journal editors, researchers, medical students, and health workers at the village level. In addition to adapting NLM databases for use in Africa, her work has encompassed a variety of media – from web-based interactive digital tutorials to posters and video. Under Ms. Royall's leadership, NLM developed greater focus on global health by piloting demonstration projects which drew strength from one another and tied into NLM’s major programs and databases
Prior to government service, she was Deputy Director of SatelLife, a nonprofit dedicated to satellite delivery of public health and medical information in developing countries. As part of the team setting up the first Internet connections for health in sub Saharan Africa, she initiated and directed the HealthNet Information Service. HealthNet News, the first electronic health publication on the continent published weekly for 20 years and pioneered digital sharing of medical literature in medical schools of sub Saharan Africa.
In 2007 - 2008, she was Fulbright Scholar to Uganda, based in the Office of the Dean at Makerere University, and has since served as a Fulbright Specialist at Kenyatta University in the Office of the Vice Chancellor.
Retired from U.S. Government service, she is currently principal investigator for the African Digital Health Library (ADHL), funded by the Office of Global AIDS Coordinator/U.S. Department of State. Based at 5 universities across Africa, ADHL will showcase in-country research previously not digitally accessible. She is also developing an African Student Innovation Fund with Carnegie Mellon University’s Africa campus in Kigali, Rwanda.
 Ronald V. Dellums
Ronald V. Dellums
A life-long advocate of peace and social justice, Ron Dellums served for 27 years as an outspoken and highly respected member of the US House of Representatives. Initially seeking a life in education, community activism and social work, Mr. Dellums was called to public office in 1967. His tenure in politics has been defined by a strong adherence to the principles of social justice, community activism and peace as viable and necessary national and international pursuits. In 2007, Mr. Dellums returned to public office as the Mayor of Oakland, California.
Born in Oakland, Mr. Dellums served in the United States Marine Corps, before going on to earn an AA from Oakland City College, a BA from San Francisco State University, and a MSW in psychiatric social work from the University of California, Berkeley. After completing his degrees, Mr. Dellums worked as a psychiatric social worker and in various anti-poverty programs. He served on the Berkeley City Council from 1967 until 1970. He then went on to win a seat to the US Congress, serving from 1971 until 1998.
Throughout his career, Mr. Dellums has brought parties together, removed obstacles, and gotten things done. He has advised world leaders on sensitive matters of global peace and national security in the Middle East, Cuba, South Africa, Haiti, and Bosnia, achieving important outcomes for the international community. At a local level, when the Port of Oakland dredging was stalled, he brought the parties together and found solutions acceptable to the previously warring interests of the Port, the environmental community, labor, and the Corps of Engineers. Similarly, Mr. Dellums was instrumental in resolving countless labor disputes, including an intractable garbage dispute between Waste Management and the Teamsters, as well as a threatened strike of 1,000 Port truck drivers.
Mr. Dellums is currently the President of The Dellums Institute: Partners for Global Health and Justice, an “action tank” to train the new generation of social justice leaders and convene non-traditional partners to work together on tackling major world problems of climate change, youth disenfranchisement, health disparities, and poverty. He also serves as Chair of the Dellums Commission on the Status of Boys and Men of Color, reconvened by the National Collaborative on Health Equity.
 Harold C. Smith, Ph.D.
Harold C. Smith, Ph.D.
Dr. Harold C. Smith, Ph.D., Professor in Biochemistry, Biophysics, and Oncology with secondary appointments as Professor in the Department of Genetics and Pathology and a Member of the Center for RNA Biology at the University of Rochester, School of Dentistry and Medicine, to the Company's Scientific Advisory Board. At the University of Rochester Dr. Smith directs a research laboratory and teaches undergraduate and medical school courses in biochemistry. He is a member of the RNA Society and a member of the American Association for the Advancement of Science. He received the SUNY at Buffalo's Distinguished Alumni Award as well as several awards from the University or Rochester for contributions to the teaching mission and leadership in mentoring. Dr. Smith has been an opinion leader in RNA biology where he established the first Gordon Research conference on RNA Editing. Dr. Smith has also been the recipient of a number of grants, including grants from the Bill and Melinda Gates Foundation, the NIH, the United States Air Force, and the Office of Naval Research. In total, Dr. Smith has received federal and foundation research grants in excess of $6.5 million. Dr. Smith has been an opinion leader in RNA biology where he established the first Gordon Research conference on RNA Editing. Dr. Smith is the author of more than 100 peer-reviewed manuscripts and reviews during his career, exceeding more than 9,973 citations.
Dr. Smith received his Ph.D. from SUNY at Buffalo, following BS and MS degrees from Purdue University and an MA degree from SUNY at Buffalo. Dr. Smith also held post-doctoral positions in biochemistry at SUNY at Buffalo followed by three post-doctoral positions in biochemistry, pharmacology, and medical genetics, respectively, at Baylor College of Medicine.
In addition, Dr. Smith is the Founder and CEO of OyaGen, Inc., a biotech company in Rochester, New York developing novel therapeutics for infectious disease and cancer. OyaGen has raised in excess of $4 million from venture and angel investor sources and two federal grants for assay development.
 Dr. Michael J. Goldblatt
Dr. Michael J. Goldblatt
Dr. Goldblatt, the Former Director of Defense Sciences at the Defense Advanced Research Projects Agency (DARPA), holds extensive experience in successfully pioneering next-generation technologies, including host-oriented therapeutics for infectious disease. He received his B.A. in Biology from Reed College and his Ph.D. and J.D. from the University of California-Davis and is admitted to practice law in New York and Washington, D.C. and with the United States Patent Bar.
Dr. Goldblatt is also the President and CEO of Functional Genetics, a privately held biotechnology Company founded in 2001. Functional Genetics focuses on the development of new antibody-based therapeutics to prevent and treat a broad spectrum of viruses including HIV, Herpes, and respiratory illnesses. Functional Genetics' leading candidate FGI-101-1A6 is a fully human monoclonal antibody which targets and eliminates cells that have been infected by various viruses including HIV-1 and influenza. FGI-101-1A6 has successfully completed its Phase IA clinical trial.
Dr. Goldblatt has over 20 years of experience working in biotechnology, product development, and regulatory affairs. He served as the Science and Technology Officer at McDonald's Corporation and Director of Scientific and Regulatory Affairs at General Foods Corporation. Dr. Goldblatt has extensive knowledge and experience in the identification and commercial development of early stage technologies.
 Richard Ogden, Ph.D.
Richard Ogden, Ph.D.
Dr. Ogden co-founded RORR Inc., a medical, scientific consulting and education company contracted by U.S. and European-based clients, including the International AIDS Society. RORR provides consulting for companies with a focus on viral infectious disease including HIV, Hepatitis C and respiratory viruses.
Beginning his prestigious career as a Post Graduate Chemist at Cambridge University, Dr. Ogden then held several positions at Agouron Pharmaceuticals including Principal Scientist/Manager, New Projects Research, and Director and Senior Director of Scientific Development. Dr. Ogden was also the Senior Director of Scientific Affairs, HIV US Medical at Pfizer Inc.
Dr. Ogden was a team member in the HIV project that led to the discovery and development of Nelfinavir (Viracept).
In working with Pfizer, he had an additional opportunity to work with the Pfizer Foundation in its support of the Academic Alliance and its efforts in Uganda, and with Corporate Philanthropy, in its support of the World Economic Forum and the Global Business Council.
Dr. Ogden received his bachelor's degree in natural sciences and doctorate in synthetic organic chemistry at Cambridge University. His academic career started with postdoctoral research studying RNA transcription and processing at the University of California, San Diego, following which he undertook independent research, funded by the National Science Foundation, in the area of protein and RNA structure-function relationships at the University of California, San Diego and the Agouron Institute.
 Dr. J. Thomas August
Dr. J. Thomas August
Dr. August’s distinguished career in clinical research directed at the molecular biology and protein structure of RNA viruses, and clinical exploration of human immunology has positioned him as a leading authority on human immune response mechanisms.
Dr. August currently holds the positions of a University Distinguished Service Professor of Pharmacology and Molecular Sciences, and Oncology at The Johns Hopkins University School of Medicine, Professor of Medicine, National University of Singapore; and Professor, Perdana University Graduate School of Medicine, Malaysia.
Dr. August has been involved with the development of a new generation of HIV vaccines and the protein antigenic structure of leading viral pathogens, including HIV-1, influenza, and other pathogens including dengue and West Nile viruses. His numerous publications are reflective of scientific commercial enterprises in cancer and HIV.
 Dr. Ritchard L. Fishman
Dr. Ritchard L. Fishman
Dr. Fishman established his practice in 1961, and since then has been seeing patients of all ages for Diabetes, Hypertension, Weight Loss, Arthritis, Pain Management and many other medical problems. Dr. Fishman is widely recognized as a leader in the research in these fields. Since 1998, Dr. Fishman has been involved in clinical trials for medications, treatments, devices and vaccines for major pharmaceutical companies seeking FDA approval. Dr. Fishman is also a Chairman of the New Life Diabetes Center’s Medical Review Board and is responsible for reviewing all medical operations supervised by this Center. Dr. Fishman graduated Ohio State University in 1953 where he earned a Bachelor of Science degree with a Major in Biology. He also received his Medical Doctor degree from Ohio State University in 1957. Dr. Fishman has professional affiliations with the Downey Community Hospital, Whittier Presbyterian Hospital, and Whittier Hospital. Also, he has been a guest lecturer with the Western University School of Osteopathic Medicine and Senior Medical advisor for New Life Diabetic Centers in California and Nevada.
 Michael McGrath, MD, Ph.D.
Michael McGrath, MD, Ph.D.
Dr. Michael McGrath is co-founder of Pathologica LLC and Neuraltus Pharmaceuticals, both privately held companies involved in developing therapeutic approaches for the treatment of various chronic diseases. Pathologica is developing a novel approach to the treatment of HIV disease, its lead drug candidate, PA-300, targeting HIV reservoirs in macrophages implicated as a cause of HIV related neurologic disease, cardiovascular disease and various forms of cancer. UCSF and Pathologica received approximately $10M from the NIH to perform all of the preclinical studies required by the FDA for administering PA300 to humans, with clinical trials targeted to begin in 2013. Dr. McGrath and colleagues raised $17M in 2009 to fund Neuraltus' phase 2 clinical trial in patients with amyotrophic lateral sclerosis using NP001 in early 2013, the first macrophage targeted approach for Lou Gehrig's disease.
Dr. McGrath maintains an extensive resume spanning more than three decades with a strong focus on HIV and oncology. Dr. McGrath's career in HIV began early in the HIV epidemic as Assistant Professor of Medicine, AIDS/Oncology Division, University of California, San Francisco/San Francisco General Hospital in 1985. In the same year he began serving as an ad hoc grant reviewer for the NIMH, NCI, NIAID and the VA and joined the Scientific Advisory Board for The American Foundation for AIDS Research (AmFAR) where he continues to serve.
Since joining the staff of the UCSF in 1985, Dr. McGrath has held numerous positions at USCF including Assistant Professor of Laboratory Medicine UCSF/SFGH, Associate Professor of Laboratory Medicine and Medicine UCSF/SFGH and Associate Professor of Pathology UCSF/SFGH.
Dr. McGrath earned his Bachelor's of Science (BS) at the University of Minnesota in 1974 in Biology. He obtained his Medical degree (MD) in 1980 at Stanford University School of Medicine and his PhD in Cancer Biology in 1985 at Stanford University School of Medicine.
 Robert Melamede, Ph.D.
Robert Melamede, Ph.D.
- Cannabis Science President Emeritus
Dr. Melamede has a Ph.D. in Molecular Biology and Biochemistry from the City University of New York. He retired as Chairman of the Biology Department at University of Colorado, Colorado Springs in 2005, where he continues to teach. Dr. Melamede is recognized as a leading authority on the therapeutic uses of cannabinoids, and has authored or co-authored dozens of papers on a wide variety of scientific subjects. Dr. Melamede also serves on the Scientific Advisory Board of Americans for Safe Access, the Unconventional Foundation for Autism, The World Aids Institute, Board Tim Brown Foundation (The Berlin Patient), Phoenix Tears Foundation, and regularly consults with professional and lay persons around the world regarding cannabis and health issues. He also served as a director of Newellink Inc, a Colorado-based company specializing in cancer research.
 Mieko Perez
Mieko Perez
- Scientific Advisory Board member
Mieko is Co-Founder of The Unconventional Foundation for Autism UF4A.ORG and is the President of CA Corporate & Attorney Services Inc. Mieko gained significant recognition for bringing her son’s success with medical cannabis to the public and birthing the significant “Joey Strain.” In 2009, Mieko decided to go public with her son's success with medical cannabis she has become a source of inspiration for other families lending her voice for unconventional and holistic treatment options within the autism spectrum. Mieko’s combined legal & special education savvy resources make her a very sought out “Warrior” for any family that contacts her. As President of CA Corporate & Attorney Services Inc., Mieko has over 15 years of being the supportive service for law firms nationwide. As Co-founder of UF4A.ORG, an informational website she now consults with families, autism organizations, universities and healthcare professionals who have exhausted all other treatments.
Mieko Perez’s efforts to help families in the autism community have helped her to achieve Congressional Recognition for her community service work. Her expertise in cannabis consulting with special needs families has made her an ideal Board Member on the NORML’s Woman’s Alliance. Mieko has now taken on the role to ensure no family will be left behind when choosing this treatment in her international position.
Mieko Perez’s efforts to help families in the autism community have helped her to achieve Congressional Recognition for her community service work. Her expertise in cannabis consulting with special needs families has made her an ideal Board Member on the NORML’s Woman’s Alliance. Mieko has now taken on the role to ensure no family will be left behind when choosing this treatment in her international Position.
]]>
 Ronald V. Dellums
Ronald V. Dellums
- Chairman
A life-long advocate of peace and social justice, Ron Dellums served for 27 years as an outspoken and highly respected member of the US House of Representatives. Initially seeking a life in education, community activism and social work, Mr. Dellums was called to public office in 1967. His tenure in politics has been defined by a strong adherence to the principles of social justice, community activism and peace as viable and necessary national and international pursuits. In 2007, Mr. Dellums returned to public office as the Mayor of Oakland, California.
Born in Oakland, Mr. Dellums served in the United States Marine Corps, before going on to earn an AA from Oakland City College, a BA from San Francisco State University, and a MSW in psychiatric social work from the University of California, Berkeley. After completing his degrees, Mr. Dellums worked as a psychiatric social worker and in various anti-poverty programs. He served on the Berkeley City Council from 1967 until 1970. He then went on to win a seat to the US Congress, serving from 1971 until 1998.
Throughout his career, Mr. Dellums has brought parties together, removed obstacles, and gotten things done. He has advised world leaders on sensitive matters of global peace and national security in the Middle East, Cuba, South Africa, Haiti, and Bosnia, achieving important outcomes for the international community. At a local level, when the Port of Oakland dredging was stalled, he brought the parties together and found solutions acceptable to the previously warring interests of the Port, the environmental community, labor, and the Corps of Engineers. Similarly, Mr. Dellums was instrumental in resolving countless labor disputes, including an intractable garbage dispute between Waste Management and the Teamsters, as well as a threatened strike of 1,000 Port truck drivers.
Mr. Dellums is currently the President of The Dellums Institute: Partners for Global Health and Justice, an “action tank” to train the new generation of social justice leaders and convene non-traditional partners to work together on tackling major world problems of climate change, youth disenfranchisement, health disparities, and poverty. He also serves as Chair of the Dellums Commission on the Status of Boys and Men of Color, reconvened by the National Collaborative on Health Equity.
 Roscoe M. Moore, Jr., D.V.M., Ph.D., D.Sc.
Roscoe M. Moore, Jr., D.V.M., Ph.D., D.Sc.
- Special Senior Advisor
Until his retirement, Dr. Roscoe M. Moore, Jr. served with the United States Department of Health and Human Services (HHS) and was for the last twelve years of his career the principal person responsible for global development support within the Office of the Secretary, HHS, with primary emphasis on Continental Africa and other less developed countries of the world. He was the principal liaison person between the HHS and Ministries of Health in Africa with regard to the development of infrastructure and technical support for the delivery of preventive and curative health needs for the continent.
Dr. Moore received his Bachelor of Science and Doctor of Veterinary Medicine degrees from Tuskegee Institute; his Master of Public Health degree in Epidemiology from the University of Michigan; and his Doctor of Philosophy degree in Epidemiology from the Johns Hopkins University. He was awarded the Doctor of Science degree (Honoris Causa) in recognition of his distinguished public health career by Tuskegee University.
Dr. Moore was a career officer within the Commissioned Corps of the United States Public Health Service (USPHS) entering with the U.S. National Institutes of Health (NIH) and rising to the rank of Assistant United States Surgeon General (Rear Admiral, USPHS) within the Immediate Office of the Secretary, HHS. He was selected as Chief Veterinary Medical Officer, USPHS, by Surgeon General C. Everett Koop.
Dr. Moore served as an Epidemic Intelligence Service Officer with the U.S. Centers for Disease Control and Prevention (CDC). He was with the Center for Veterinary Medicine, U.S. Food and Drug Administration (FDA), before becoming Senior Epidemiologist within the National Institute for Occupational Safety and Health, CDC. He served as the Chief Epidemiologist with the Center for Devices and Radiological Health, FDA. He directed the Epidemiology and Biostatistics Program and was an Assistant Professor of Oncology within the Howard University College of Medicine Cancer Center.
 Melvin P. Foote
Melvin P. Foote
Mr. Melvin P. Foote is a pioneer in the field of African Affairs. He has over 35 years of experience and has worked in over 30 African countries. And he is recognized as a leading expert on issues related to African policies and programs.
Mr. Foote founded the Constituency for Africa (CFA) in 1990, in order to establish a network of organizations, groups and individuals committed to the progress and empowerment of Africa and African people worldwide. CFA’s mission is to build public and private support for Africa, and to help shape a progressive U.S. policy towards Africa.
Mr. Foote is also the founder of a dynamic CFA-related program called the African American Unity Caucus (AAUC). The AAUC is a broad-based coalition of African-American and African leaders of Africa-focused organizations and groups, to promote pan-Africanism and to link Diaspora leaders in the Western Hemisphere with the African Union (AU), the coordinating forum of the nations of Africa. Further, Mr. Foote serves as an advisor to the African Union’s Ambassador to Washington and as a consultant to the World Bank on African Diaspora issues.
Mr. Foote has participated in numerous high-level missions to Africa, including as a participant on a White House delegation to assess the genocide in Rwanda in 1994; as part of a high profiled team that sought to end the war between Ethiopia and Eritrea in 1998; as a member of a Presidential mission to five African countries in 1998 to promote U.S. trade with Africa and the African Growth and Opportunity Act; as the leader of a mission to reach a comprehensive peace agreement in Sudan in 2001; and as the leader of a delegation to South Africa in 2003 to assess the New Partnership for African Development (NEPAD), a continent-wide initiative to promote economic integration and development.
Mr. Foote is the recipient of numerous awards and recognitions that include the “Order of the Lion” Award in 1998, which is the highest public service honor of the Government of Senegal, and the Congressional Black Caucus 2001 Annual Legislative Conference’s Diggs Award for Foreign Affairs in recognition of his outstanding commitment and achievement on issues and concerns pertaining to Africa. Mr. Foote is a regularly requested speaker on radio and television as well as a prolific writer of articles and editorials featured in newspapers and magazines across America.
Prior to founding CFA, Foote served as a U.S. Peace Corps Volunteer and teacher in Ethiopia and Eritrea from 1973-1976. From 1981-1984, he served as Africare’s Representative in Somalia. From 1984-1994, Mr. Foote worked in Africare’s Washington headquarters as their Director of Constituency Development, which led to the founding of CFA.
 Julius Garvey
Julius Garvey
Julius Garvey, M.D., F.A.C.S, F.R.C.S.(C), F.I.C.S., F.A.C.Ph., F.A.C.C.W.S. is a Board-Certified surgeon specializing in the diagnosis and treatment of vascular diseases. As the founder and Medical Director of Garvey Vascular Specialists, Dr. Garvey brings a wealth of experience and knowledge into our practice. His skill and proficiency provide our patients with an extensive range of expertise in the areas of arterial, venous diseases.
Dr. Garvey, a highly trained and credentialed surgeon, has held extensive academic appointments. He has worked as an Associate and Assistant Professor of Surgery at Albert Einstein College of Medicine in Bronx NY, Assistant Professor of Surgery, State University of NY at Stony Brook, NY, as well as Instructor in Surgery at both Albert Einstein College of Medicine in Bronx, NY and Columbia University College of Physicians & Surgeons, NY. Additionally, his hospital appointments are expansive. He has been an Attending Cardiothoracic Surgeon at Harlem Hospital Center in NY, NY and Montefiore Hospital in Bronx, NY, Associate Attending Head of Thoracic Surgery at the Montefiore Morrisania Affiliate in Bronx, NY, and Acting Program Director in the Division of Cardiothoracic Surgery at Long Island Jewish Medical Center in New Hyde Park, NY. He was also Chief of Vascular & Thoracic Surgery at Queens Hospital Center and is presently an attending surgeon on staff of the Long Island Jewish Medical Center.
Dr. Garvey is both nationally and internationally recognized for his contributions to medicine and speaks, teaches and presents clinical research at conferences across the country. He is a Member of the International College of Angiology, the Phlebology Society of America, the NY Academy of Sciences, the American Heart Association, the Society of Thoracic Surgeons, the Association for Academic Surgery, the Association for the Advancement of Wound Care and the NY Society of Thoracic Surgery.
Dr. Garvey is a Fellow of the Royal College of Surgeons (Canada), the American College of Surgeons, The American College of Phlebology, the International College of Surgeons, and the American College of Chest Physicians. In addition, he is a Diplomate of the Board of Cardiothoracic & Vascular Surgery, the American Board of Surgery, and the American Academy of Wound Management. Dr. Garvey’s exposure and expertise in vascular surgery, cardiovascular surgery, Phlebology, and wound care leads his determination to comprehensively address the concerns of all patients seen at Garvey Vascular Specialists afflicted with vascular disorders.
Click here to read Dr. Garvey's Curriculum
 Allen A. Herman, M.B., Ch.B., Ph.D.
Allen A. Herman, M.B., Ch.B., Ph.D.
Dr. Allen Herman is an epidemiologist and public health physician. He was the founding Dean of the National School of Public Health, Medical University of Southern Africa of the Republic of South Africa. He graduated in Medicine from the University of Natal in 1977 and completed his doctoral work in Epidemiology at the University of the Witwatersrand in 1989. He was a postdoctoral fellow in Epidemiology at Columbia University in New York in 1986 and a member of the faculty from 1987 to 1988. From 1989 to 1997 he was a Visiting Scientist at the National Institutes of Health. He was an Adjunct Professor at the George Washington University School of Public Health and Health Services from 1997 to 2004. Dr. Herman has extensive experience in developing and managing epidemiologic and public health research projects, and he is adept at secondary data analysis. In 1990 he helped develop the Baltimore Project, an Infant Mortality Reduction Initiative in Baltimore, Maryland. This community-based, model enriched prenatal care demonstration project for East Baltimore formed part of the basis for a $160 million dollar federally funded national program to reduce infant mortality. In 1992 he developed the scientific basis of the National Institutes of Health - District of Columbia Infant Mortality Reduction Initiative. He was the scientific director of this $25 million community-based U.S. federal research project that was designed to identify the critical factors that contribute to a high infant mortality among poor inner city African Americans and to develop interventions to reduce infant mortality rates.
Dr. Herman was effective in harnessing the energies of South Africans and Americans to fight HIV/AIDS in Africa. He led the development of the Bristol-Myers Squibb Company Secure the Future Project, a $100 million program that focused on the health of women and children infected and affected by HIV. He was the Advisor to then Chairman Ronald V. Dellums, who served President Bill Clinton (during his second term) as the Chairman of the U.S. Presidential Committee on HIV/AIDS on HIV/AIDS in Africa. Dr. Herman, Mr. Dellums and Congresswoman Barbara Lee (the founding cochair of the Congressional HIV/AIDS Caucus) developed the idea of an “AIDS Marshall Plan for Africa” which was transformed into the Global Fund to fight AIDS, Tuberculosis and Malaria, which led in 2003 to the President’s Emergency Plan for AIDS Relief (PEPFAR) and its reauthorization in 2008. Dr. Herman was the director of the Secure the Future Public Health Fellowship Program that trained more than 150 fellows in dealing with AIDS. This is the largest such training program in Africa, and his students included the Minister of Health of Swaziland, the Director of the AIDS Program of Lesotho, and the Directors of the AIDS Programs from a number of South African provinces. He also worked as the advisor to the South African National Defense Force and helped bring a substantial antiretroviral treatment program to the South African military. This program was designed to bring treatment through the use of clinical trials to a large population of military families and was designed to enable the South African government to objectively create programs of treatment for the large number of infected South Africans. For his work in HIV/AIDS, Dr. Herman was awarded the Heroes in Medicine award by the International Association of Physicians in AIDS Care.
Click here to read Dr. Herman's Curriculum Vitae
 Darren Parker
Darren Parker
Darren Parker has served as a Public Servant, Business owner and influential leader for more than 30 years. He has been honored numerous times by both the President pro Tempore(s) of the Senate and California State Assembly Speaker(s). He has also been appointed to various boards and agencies including the City of Lancaster’s Citizens General Plan Advisory Commission by Mayor Hon. Bishop Henry Hearns as well as the Antelope Valley Fair Board appointed by Governor Gray Davis and Governor Jerry Brown. As a resident of the Antelope Valley, for over 25 years, Darren worked with government agencies, nonprofit organizations, and businesses to better the community. Darren is one of the founding members of the Antelope Valley Human Relations Commission and was elected President for 18 consecutive terms. Darren has focused on education, equal rights, public safety, smart business growth, improving the quality of life for our Veterans, green energy and agriculture.
Mr. Parker was nominated and appointment Chairman by Lancaster mayor, R. Rex Parris, to the city of Lancaster’s Architectural Committee. Darren has also served on the Antelope Valley College Citizens Oversight Review Commission as Chairman, Antelope Valley NAACP as President and the Los Angeles County Commission for the Department of Children and Families as the Vice Chairman. Darren is serving his 6th term as elected statewide Chairman of the California Democratic Party’s African American Caucus and has worked with the last 3 Presidents of the United States on varies issues facing local government and education, including the “My Brother’s Keeper” program with President Obama. Parker continues to serve as the elected CA State executive board member for the 36th AD and as the Los Angeles County Democratic Party Region 1 Secretary.
Darren also serves on the Eastside School District Advisory Council, Antelope Valley Union High School District Advisory Council, and Palmdale School District African American Advisory Council and is an AVID (Advanced via Individual Determination) Community Liaison and raised funds to support safe schools and other causes.
On the other side, Darren served as an active member in the Communication Workers of America Union during his 30-year career at AT & T. He was appointed to the Executive Board, elected Vice President. He is now a CWA Lifetime Retiree.
As a former small business owner of The Parker Group, LLC, Darren is known for his track record of excellence and achievements. He understands that leadership requires the capability to fully access and understand the issues at hand, communicate a clear vision and the ability to pull people together to accomplish a common goal.
 Jacques P. Walker
Jacques P. Walker
Mr. Walker is an experienced entrepreneur with over twenty years of experience successfully developing, managing, and executing commercial initiatives in the United States and in emerging markets worldwide. In addition to his work in the U.S., Mr. Walker has focused extensively on markets in Sub-Saharan Africa, and has worked in over 18 African countries.
Mr. Walker serves as a Senior Advisor to the Constituency for Africa (CFA), one of the leading organizations in the United States committed to educating and mobilizing the U.S. public on matters pertaining to Africa and the African Diaspora. For CFA, Mr. Walker helps plan, develop, and manage major CFA initiatives, including the annual Ronald H. Brown African Affairs Series. Mr. Walker actively engages with CFA’s sponsors and stakeholders in the public sector, private sector, civil society, academia, and the media.
Mr. Walker co-founded and serves as Chief Operations Officer of an integrated energy company with operations in the United States, the Middle East, and Sub-Saharan Africa. Mr. Walker’s responsibilities include helping shape overall corporate strategy, managing the company’s operations, directing the company’s Oil & Gas Business Unit, developing and managing multiple strategic partnerships, and providing client support services. Mr. Walker has helped the company win multiple projects, including over $4 billion in Indefinite Delivery/Indefinite Quantity (IDIQ) contracts.
Mr. Walker previously worked as a principal and senior consultant with an international consulting firm in Washington, DC. Mr. Walker’s responsibilities included developing corporate strategy, managing the company’s day-to-day operations, and managing the company’s international project portfolio. Mr. Walker provided clients with market analysis, economic analysis, risk analysis, and business development services in emerging markets, with a particular focus in Sub-Saharan Africa. His clients included the world’s largest Oil & Gas engineering, procurement, and construction (EPC) contractor, and one of the world’s largest project management/infrastructure development firms.
Mr. Walker previously co-founded and served as Chief Executive Officer of a consulting firm that provided strategic advisory services, management consulting, business development, and project development services to public and private-sector clients globally. One of Mr. Walker’s clients was a manufacturer of generic and essential drugs, including analgesics, anti-allergics, anti-bacterials, anti-malarial, antihelminitics, and other pharmaceutical and botanical products on the World Health Organization’s essential drug list. At the time of Mr. Walker’s engagement with this client, this pharmaceutical company had successfully registered over 24 products in almost 20 African countries. In an effort to help this company expand its operations throughout Africa, Mr. Walker worked as a member of a team of consultants to structure a $250 million, three-party Memorandum of Understanding (MOU) with the pharmaceutical company, the Export-Import Bank of the United States (Ex-Im Bank), and the Republic of Ghana. This MOU was part of Ex-Im Bank’s $1 billion program to support Africa's fight against HIV/AIDS. Mr. Walker graduated with a B.A. in Economics from the University of Maryland at College Park.
]]>

AUTHORIZED SHARES
Common - 3,000,000,000
Common, Class A - 100,000,000
Preferred - 1,000,000
SHARES OUTSTANDING
Common - 2,327,855,296
Common, Class A - 0
Preferred - 1,000,000
CBIS TRANSFER AGENT
Securities Transfer Corporation
2901 N Dallas Parkway
Suite 380
Plano, Texas 75093
Telephone: (469) 633-0101 ext. 113
Fax: (469) 633-0088
Website: www.stctransfer.com